Some reports claim that as many as 90% of adverse drug reactions go unreported. Will moves to widen the net of pharmacovigilance fill in the gaps or will it point to previously unsuspected ADRs?
Not all adverse drug reactions are obvious to those suffering them. A glance at the documentation placed with any prescription drug can reveal symptoms ranging from a mild sense of unease to death – although the two may well be related.
The problem is not restricted to patients – medical professionals too routinely under-report ADRs. In November 2014, I wrote about a Brazilian study that suggested lack of training may be partially to blame.
The problem is that it’s pretty hard to educate the entire world of patients in what really should be reported. Making it easier to report side-effects is a vital step but is it going to make much of a dent in the 90% of unreported ADRs?
I don’t think so.
Some ADRs have been almost inadvertently reported – by social media. This may provide a valuable additional strand of data. In 1997, a man taking HIV medication reported a change in the shape of his navel in a patient internet forum. Others shared their experiences and thus the ADR known as “Crix belly” was born.
This particular symptom only appears after relatively long-terms use of crixivan – longer than the duration of the clinical trials. And this highlights one of the real advantages of post-marketing reports of ADRs.
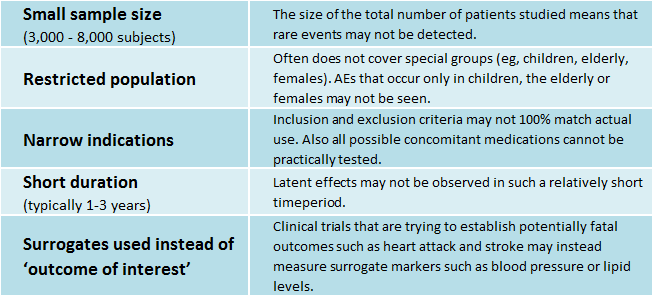
This table is taken from Whitehall Training’s online course, Pharmacovigilance I – Drug Safety and shows some of the potential pitfalls in basing ADR reporting on the information from clinical trials.
So what would the effect of casting the pharmacovigilance even wider than currently happens?
Far from alerting people to the real risks of modern drugs, it will probably have the effect of increasing the list of relatively non-serious reactions. After all, the kinds of grotesque reactions that litter the internet are hardly going to be ignored by the majority of sane people. It is the small and potentially embarrassing things that go unnoticed – like a belly button suddenly switching from an “innie” to an “outie.”
It will be fascinating to look at drug labels in the next decade.