India has been vocal about investment in its pharmacovigilance programme over the last year. However, despite being a US$ 3.1 billion market for medical devices, it has no equivalent process for registering adverse events caused by medical devices or for tracking their safety record. Instead it relies on data from other countries.
The proposed plan, involves a nationwide programme, involving district hospitals, medical colleges and corporate hospitals. The intention is to introduce the system in three months.
The collection of medical device associated adverse event data will initially be done by ten medical colleges throughout the country. The biotechnology wing of the Sree Chitra Thirunal Institute of Medical Sciences and Technology in Thiruvananthapuram will act as the national collaborating centre.
The US has led the world in device vigilance – requiring that all related adverse events be reported to the FDA for over 30 years. Since 1984, there have been many pieces of US legislation and guidance aimed at controlling the way adverse events are tracked and acted upon. The most recent was the FDA’s Draft Guidance on Medical Device Reporting for Manufacturers, published in July 2013.
The cornerstone of medical device reporting in Europe was the EC Directive on Medical Devices, 93/42/EEC (EC 93a). Among other things, this defined the term “safety” in relation to devices.
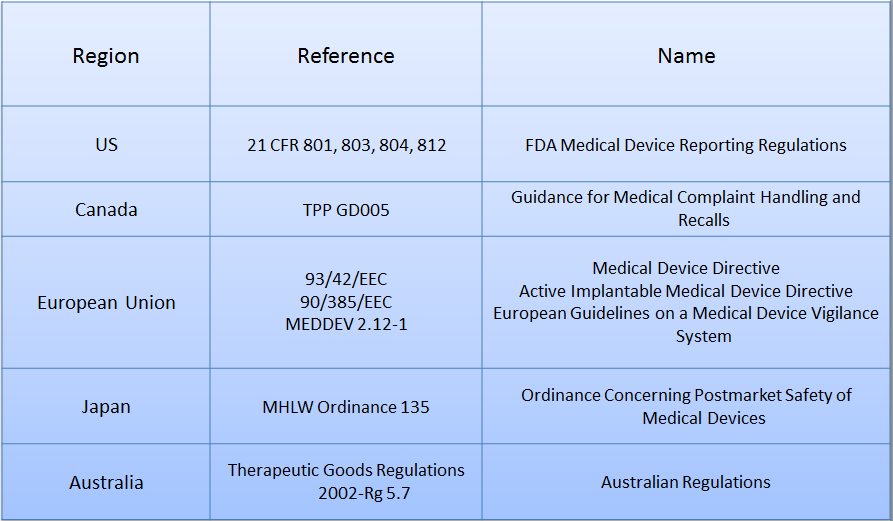
The following table summarises the key legislation throughout the world and is taken from the Whitehall Training online training course, Medical Device Safety & Vigilance.