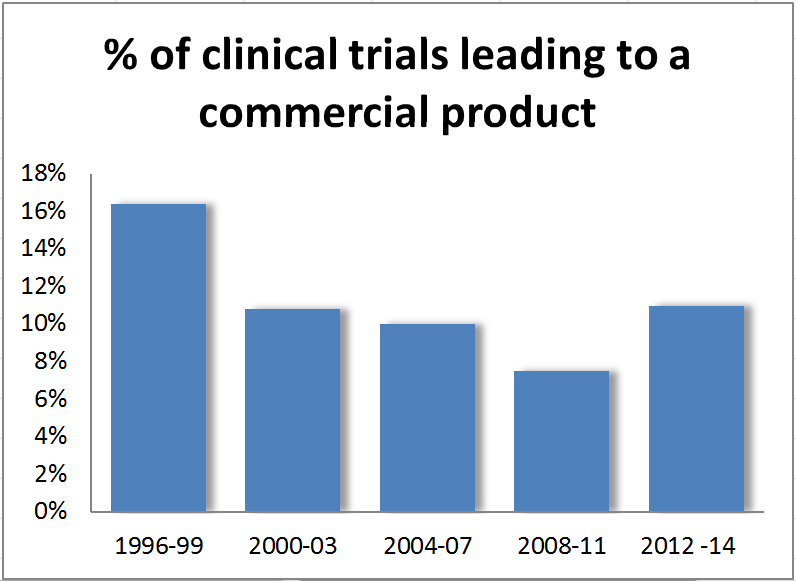
It goes without saying doesn’t it – the search for new drugs is becoming harder and harder. Why then does the percentage of successful trials seem to be growing?
Bucking the trends of recent years, a recent report by McKinsey & Co shows that 11% of clinical trials between 2012 and 2014 were successful – the first increase for at least 20 years.
Why is this? Have we finally cracked what has been holding us back? Have recent scientific advancements started to pay dividends? Or is something else acting here?
The results were published last month in Nature Reviews Drug Discovery, and came from an examination of more than 9,200 novel compounds that were developed from 1996 to 2014.
The consultants put some of the blame for the pre-2012 declines on industry cutbacks. Between 2007 and 2010, a number of mergers and acquisitions caused drug makers to cut back on R&D via “pipeline pruning.”
This concentration of resources on only certain medical conditions led to higher quality product pipelines the report argues. Of course, the flip side of this is that certain less profitable diseases received fewer new drugs.
During this period, the proportion of compounds failing in Phase I and early clinical trials actually rose – perhaps implying that companies are running more thorough evaluations and doing so sooner.
This certainly seems backed up by the number of Phase II and III trials leading to product registration. In 2012 to 2014, the likelihood was 64% - up from 60% in the previous period. The odds of a Phase II trial progressing to Phase III also rose.
There have been no major changes to the rules of Good Clinical Practice (GCP) that could explain this. The vast majority of the world’s trials still follow the basic rules laid down by the ICH.
The report does suggest that some of this boost could be a result of the fast-tracking approach being applied to experimental drugs used to treat certain conditions. The risk with this approach is that it may not increase the likelihood of success – it may just move failure back to a later and far more expensive trial stage.
I have written before about outcome switching – and I can’t help wondering if this may have a part to play in the figures too. If researchers are becoming more canny about what defines “success,” this could certainly have a role.
Whatever the combination of factors that has led to this increase, it’s too early to know if this is the start of a new trend or just a blip. However, it does provide a bit of potentially good news in the clinical trials sector.
Let’s hope that there isn’t a shift towards late Phase trials with human participants unless combined with greater late phase success – otherwise, I question whether the shift is really following the over-riding pillar of Good Clinical Practice – that the health and safety of the participants must be protected at all times.
Whitehall Training’s online GCP Courses are used to train thousands of workers in the world’s most high profile clinical trials. You can learn more about the courses [here]